|
| |
|
Are there fitness advantages associated with a large inflorescence in Gymnadenia conopsea ssp. conopsea?
|
Elisa Vallius, Sanna Arminen and Veikko Salonen
|
|
Department of Biological and Environmental Science, University of Jyväskylä, P.O. Box 35, FIN-40014 University of Jyväskylä, Finland; e-mail:

|
| |
|
Abstract
We studied the relationships between inflorescence size and pollinator attraction, female reproductive success and seed predation in Gymnadenia conopsea ssp. conopsea (L.) R. Br., a threatened terrestrial orchid species. We also conducted a hand-pollination experiment to examine the effect of geitonogamous pollination on seed production. We found that plants with a large inflorescence attracted more flower visitors and produced more seed capsules than plants with fewer flowers. The duration of butterfly visits, the number of visited flowers per inflorescence or the probability of seed predation did not differ between large and small inflorescences. Moreover, seed set following enforced geitonogamous pollination did not differ from that following cross-pollination. As large size of inflorescence appeared not to entail any immediate costs, we conclude that at least when examination is restricted to one season, having a large inflorescence is clearly advantageous for G. conopsea.
|
|
Introduction
Large inflorescences tend to be more attractive to pollinators than small ones (e.g. Fægri and van der Pijl, 1979; Pellmyr, 2002). A high visitation rate increases pollen movements between plants which may increase their reproductive success (e.g. Ohara and Higashi, 1994). Therefore, plants might be expected to produce as many flowers as possible depending on the resources available for reproduction. In many species, however, inflorescence size is limited by other factors than resource availability. A smaller inflorescence size may be favoured by natural selection if plants with a larger inflorescence would suffer from any of the following things decreasing fitness: (i) higher probability of geitonogamous self-pollination (Schoen and Dubuc, 1990; Harder and Barrett, 1995; Vrieling et al., 1999), (i) more severe herbivore attack (Ehrlén, 1997) or (iii) increased pre-dispersal seed predation (Molau et al., 1989; Brody and Mitchell, 1997; Leimu et al., 2002). Thus interactions between plants and their pollinators are often more complicated than usually noticed in papers on plant-animal interactions (Herrera, 2000). Furthermore, avoidance of the negative effects of attractiveness may be a stronger selective force than an increased visitation rate following allocation to advertisements (see Pellmyr, 2002 for a review).
In plants with one large inflorescence, the costs of producing an additional flower diminish with inflorescence size (Schoen and Dubuc, 1990). Therefore it may be advantageous for a plant to produce many flowers, especially if large inflorescences are favoured by pollinators (Ohara and Higashi, 1994). Yet studies with nectar-producing orchids have yielded conflicting results. A positive correlation between inflorescence size and capsule production has been shown in some species, e.g. Platanthera bifolia (L.) L.C.M. Richard (Mattila and Kuitunen, 2000). This positive effect of flower number may be due to other factors (e.g. resource availability) and therefore may not be found every year (Rodríguez-Robles et al., 1992). In milkweeds (Asclepias L.), which, like orchids, have aggregated pollen (pollinia), an increase in inflorescence size leads to higher pollination success both in terms of pollinarium removal and pollen insertion (see Wyatt and Broyles, 1994 for a review). In contrast to these results, seed set decreased as flower number increased in Epidendrum exasperatum Rchb., a self-compatible butterfly-pollinated orchid species (Calvo, 1990). Differences in the effects of inflorescence size on pollinator attraction may result from differences in flowering phenology. Moreover, Barrett et al. (1994) argued that the number of flowers open simultaneously (daily inflorescence size) may be more important for pollinator attraction than the total number of flowers in an inflorescence.
A large inflorescence may also have negative effects on plant reproductive success due to geitonogamy (i.e. pollination with pollen from the same plant) (de Jong et al., 1993). The probability of geitonogamous pollination increases with an increase in number of approaches by visitors and longer visitation sequences of pollinators on plants with many flowers (Hessing, 1988; Klinkhamer and de Jong, 1993; Johnson and Nilsson, 1999; Vrieling et al., 1999). Duration of visitation on one inflorescence is also affected by density of flowering plants in the foraging area. To avoid flight costs, pollinators may visit more flowers per inflorescence in a sparse patch of food plants than in a dense patch (Cibula and Zimmerman, 1984; Klinkhamer and de Jong, 1993). Probability of geitonogamous pollination is thus predicted to be highest for plants with a large inflorescence living in a population with sparse flowering individuals.
Pollinator movements within an inflorescence lead both to self-pollinations and to a reduction in the amount of exported pollen, so called pollen discounting (Harder and Barrett, 1995). The severity of the negative effects of geitonogamy depends on the genetic structure of the population and on the level of self-incompatibility of the species (de Jong et al., 1993). In self-incompatible milkweeds, geitonogamy may restrict fruit set to as little as 1% (Wyatt, 1981). In self-compatible species, geitonogamy has been shown to cause a decrease in seed quality (Hessing, 1988). In small and isolated populations, the production of selfed progeny as a result of geitonogamous pollination, may accelerate the loss of genetic variation (Raijmann et al., 1994).
Risk of pre-dispersal seed predation has been shown to increase with increasing flower number in many species (e.g. Molau et al., 1989; Leimu et al., 2002). The advantage of higher seed set resulting from higher pollinator visitation rate may thus be lost, because seed predators are triggered by the same attraction cues as pollinators (Molau et al., 1989) or attracted by large size of fruits produced after successful pollination (Herrera, 2000). Butterflies have been shown to be able to detect physiological differences between plants while ovipositing, and therefore a plant with a large inflorescence would be attractive to females searching for a food plant suitable for caterpillars (Myers, 1985). A large inflorescence may also attract nectar-robbing insects in addition to pollinator species thus increasing the costs of pollinator attraction (Goulson et al., 1998). Moreover, insects visiting flowers often carry fungal spores causing plant disease, and consequently, a higher visitation rate by them may lead to a higher risk of fungal disease for a plant with a large inflorescence (e.g. Shykoff and Bucheli, 1995).
In this paper, we examine the effects of inflorescence size on pollinator visitation rate, capsule set and probability of pre-dispersal seed predation in the threatened terrestrial orchid species Gymnadenia conopsea ssp. conopsea (L.) R. Br. We also discuss the relationship between geitonogamous pollination and reproductive success in isolated populations, and life-time reproduction of this species which is reproductively wholly dependent on pollinator visits.
Materials and methods
Study species and study site
Gymnadenia conopsea is an insect-pollinated perennial herb occurring in open habitats usually on calcareous soil (Pridgeon et al., 2001). In Finland, G. conopsea ssp. conopsea has become a rare taxon due to modernization of agricultural methods. In central Finland, this species can be found only in very small populations (usually only with a few flowering individuals), and the populations are located far apart (Fig. 1). Gymnadenia conopsea (subspecies conopsea in particular) is protected by law in the southern parts of Finland.
Flowering G. conopsea plants produce one flower stalk with over 15 pinkish- or reddish lilac (sometimes purple or white) flowers in a long and narrow inflorescence (Moore, 1980). Flowers open sequentially from the lowest flower up. Individual flowers remain fresh for several days, and consequently the number of receptive flowers increases during the flowering period until the first-opened ones begin to wither (S. Arminen, pers. obs.). The flowers have a long (11-18 mm; Moore, 1980) narrow spur containing nectar, and pollinating insects, mainly butterflies and hawk-moths, are also attracted by a sweet scent (Pridgeon et al., 2001, p. 298). Pollen of G. conopsea flowers is aggregated to two pollinia, which attach to the proboscis of visiting butterflies. Day-active butterfly and moth species including Aglais urticae, Ochlodes venatus (Hansen and Olesen, 1999) and Zygaena lonicerae (Nazarov and Buchsbaum, 2004) are known to visit G. conopsea flowers.

Fig. 1. Gymnadenia conopsea ssp. conopsea population in central Finland. Photo was taken by Veikko Salonen
We conducted this study in the second largest known G. conopsea ssp. conopsea population in central Finland (98 and 34 flowering plants in 1999 and in 2003, respectively), in Haapamäki, Keuruu. The study site is grassland located between a railroad bank and a small lake (Lake Niemelänjärvi). The area is kept clear from excessive shading by regular cutting of trees and bushes. Other species occurring in the study area are Betula pendula Roth, Populus tremula L., Salix phylicifolia L., Vaccinium vitis-idaea L., Potentilla erecta (L.) Räuschel, Anthoxanthum odoratum L., Deschampsia flexuosa (L.) Trin., and Calamagrostis epigejos (L.) Roth. This study was conducted after receiving a permission from the regional Environmental Centre (0999L0263/254) to work on this protected plant species and after a permission from the railroad authorities (Ratahallintokeskus) to use the railroad area during the study.
Experiments
In summer 1999, we marked all flowering G. conopsea plants with numbered sticks and covered most of the inflorescences with mesh bags before anthesis to exclude pollinators. When the plants had inflorescences in flower bud phase, we counted the flowers and we selected nine pairs of plants for the monitoring of pollinators. These nine pairs consisted of plants with as similar height and flower coloration as possible, but differing in the number of flowers. The number of flowers in the small inflorescence was considerably lower than in the large inflorescence in the same pair (Table 1). Among these nine pairs, the two plants grew less than 50 cm apart. To increase the number of plant pairs to 15, we dug up 12 plants to form six additional pairs with a similar distance (< 50 cm) between them. These six pairs were transplanted within the natural population. We removed the mesh bags just before the beginning of pollinator monitoring.
We observed pollinator visits to the formed pairs during daytime (07-19h) in 30 min. periods between 30.6.-10.7.1999. We repeated the observation periods on several days, so that each pair was monitored altogether for 2.5-4 hours. The observations ended when the first flowers in any of the plants in a pair started to wither. Observations were made at a distance of 1.5-2 m. All insect visitors were counted and classified as (i) syrphid flies (Syrphidae), (i) other flies (Diptera), (iii) butterflies (Lepidoptera), (iv) bumblebees (Bombus L. sp.), and (v) other taxa. Duration of each butterfly visit from the landing on the first flower to the departure from the last visited flower was measured with a stopwatch. The number of flowers visited during observations was counted. Visits shorter than two seconds and those longer that 120 seconds were excluded from the analyses. The extremely short visits were excluded because they did not lead to an actual feeding behaviour, and the very long ones because butterflies rather spent most of their time resting or grooming instead of actively searching nectar from the flowers.
We conducted a hand-pollination experiment to examine the possible effects of geitonogamous pollination on seed production of G. conopsea. We used ten plants each covered with a mesh bag to exclude pollinators. We pollinated 5-7 flowers in each inflorescence with one pollinium per stigma. The pollinia used were from flowers in the same inflorescence. Another group of 5-7 flowers in the same inflorescence was pollinated with one pollinium per flower from another plant in the population (cross-pollination). We marked the differently treated flowers with coloured plastic rings.
Measurements
We counted the number of opened and withered flowers and the number of capsules produced by each flowering plant in the population. We collected the flower heads from 30 monitored plants and from each of the ten plants used in the hand-pollination experiment. We dried the capsules at 70°C for 48 hours. The seeds in some of the capsules had been predated by caterpillars and we excluded these predated capsules from weighing.
We opened the dried capsules from each study plant and pollination treatment in hand-pollination experiment in separate glass tubes and placed the seeds on a microscope glass for counting of embryonic seeds. We counted 200 seeds from each sample and classified them as either embryonic or non-embryonic (i.e. in-viable without an embryo or with an embryo smaller than 2/3 of the width of the seed). We calculated the proportion of embryonic seeds for each of the samples.
In 2003, additional data on capsule production and seed predation were collected from the same population. We found 34 flowering G. conopsea plants in a slightly different position (about 50 m from the 1999 study site). We counted the number of flowers and capsules produced by each plant. We also examined the capsules for possible pre-dispersal seed predation.
Statistical analyses
We tested the paired data using Wilcoxon non-parametric test to compare flower visits, capsule set and seed predation between the small and large inflorescences which were observed at the same time. Duration of butterfly visit was not tested pair-wise because of a low number of observations. Instead this variable was tested by one-way ANOVA or by Mann-Whitney U-test, when appropriate. The relationship between flower number and number of capsules produced was tested using linear regression with year of the data collection as additional factor. Linear regression could not be used to test the relationship between flower number and relative capsule production, because non-normality of data from the year 1999. Therefore we used non-parametric Spearman rank correlations separately for each year, instead. Wilcoxon test was used to compare seed production following geitonogamous and xenogamous pollination within inflorescence.
|
Results
Large inflorescences received more insect visits during the monitoring than smaller inflorescences (Wilcoxon: n1,2 = 15, Z = -2.64, P = 0.008, Fig. 2). Most of the visitors (92.4%) were flies which clearly favoured larger inflorescences (n1,2 = 15, Z = -3.15, P = 0.002, Fig. 2). Larger inflorescences also attracted more syrphid flies than the smaller ones (n1,2 = 15, Z = -2.55, P = 0.011, Fig. 2). Butterflies seemed to favour larger inflorescences, but this result was statistically slightly insignificant (n1,2 = 15, Z = -1.95, P = 0.051, Fig. 2). Only five bumblebees visited the plants during the monitoring, and no favouring behaviour was found (n1,2 = 15, Z = -1.069, P = 0.285). Visitation rate of other taxa, mainly of coleopterans, was also not affected by inflorescence size (n1,2 = 15, Z = -1.51, P = 0.13, Fig. 2). Inflorescence size did not affect the total duration of butterfly visit (meanlarge = 13.4, meansmall =14.0, Z13,7 = -0.914, P = 0.36, Fig. 3) or the total number of visited flowers per visit (meanlarge = 2.53, meansmall = 2.63, F17,8 = -0.007, P = 0.93).
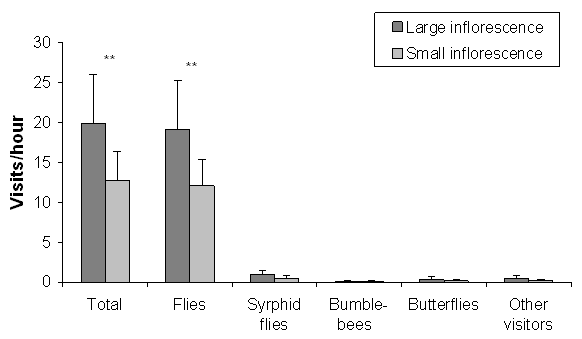
Fig. 2. Mean number of insect visits (+ SD) to large and small inflorescences in a central Finland population of Gymnadenia conopsea ssp. conopsea. * P < 0.05, ** P < 0.01
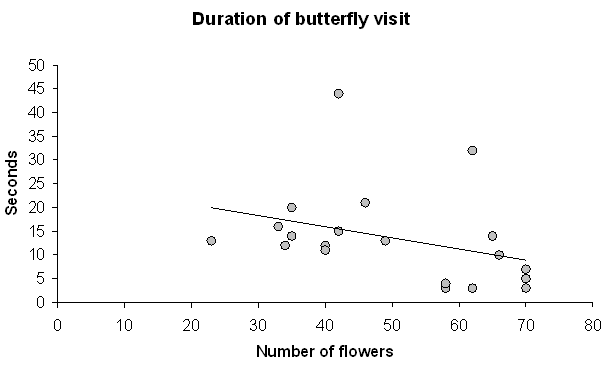 Fig. 3. Relationship between inflorescence size and number of capsules produced in two years in a central Finland population of Gymnadenia conopsea ssp. conopsea.
Fig. 3. Relationship between inflorescence size and number of capsules produced in two years in a central Finland population of Gymnadenia conopsea ssp. conopsea.
In a pairwise comparison of the monitored plants, large inflorescences produced more capsules overall than small inflorescences (Table 1). Total mass of capsules produced by the individuals with a large inflorescence was higher than that produced by the plants with a small inflorescence, and individual capsules were also heavier in the plants with a large inflorescence (Table 1). There was a trend for more capsules to be predated in plants with a large inflorescence than in plants with a small inflorescence, but the proportion of predated capsules did not differ between the different-sized inflorescences (Table 1). In 2003, only four out of 34 inflorescences were attacked by seed predators (caterpillars of Syndemis musculana Hübner, Microlepidoptera).
Table 1. Differences in flower number, capsule production, capsule weight and seed predation between Gymnadenia conopsea plants with large and small inflorescences (Wilcoxon signed ranks test, n1,2 = 15).
|
|
|
Inflorescence size |
Z |
|
P |
|
large |
|
small |
|
| Number of flowers |
57.7 |
33.9 |
-3.41 |
0.001 |
| Total number of capsules |
47.1 |
22.7 |
3.41 |
0.001 |
| Relative capsule production (%) |
81.9 |
63.8 |
3.01 |
0.003 |
| Total mass of capsules (mg) |
321 |
105 |
3.41 |
0.001 |
| Average capsule weight (mg) |
7.0 |
4.4 |
2.90 |
0.004 |
| Number of predated capsules |
5.5 |
2.5 |
1.73 |
0.084 |
| Proportion of capsules predated (%) |
10.6 |
8.0 |
0.97 |
0.334 |
|
Number of capsules produced by a plant increased linearly with increasing inflorescence size (Linear regression: t = 11.40, P < 0.001, Fig. 4) The total capsule production was lower in 2003 than in 1999 (t = -4.41, P < 0.001, Fig. 4). Large inflorescences also produced relatively more capsules in 1999 (rs = 0.406, N = 64, P =0.001), but not in 2003 (rs = -0.24, N = 33, P = 0.178) (Fig. 5). Capsules produced after geitonogamous and xenogamous pollination differed neither in dry weight (Wilcoxon: n1,2 = 10, Z = -0.357, P = 0.721) nor in the proportion of embryonic seeds (n1,2 = 9, Z = -0.770, P = 0.441).
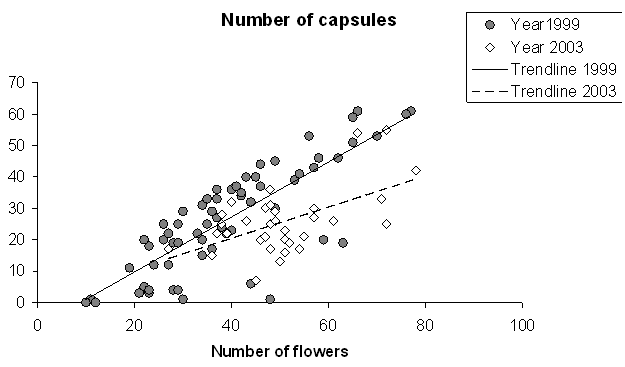 Fig. 4. Relationship between inflorescence size and relative capsule production (%) in two years in a central Finland population of Gymnadenia conopsea ssp. conopsea
Fig. 4. Relationship between inflorescence size and relative capsule production (%) in two years in a central Finland population of Gymnadenia conopsea ssp. conopsea
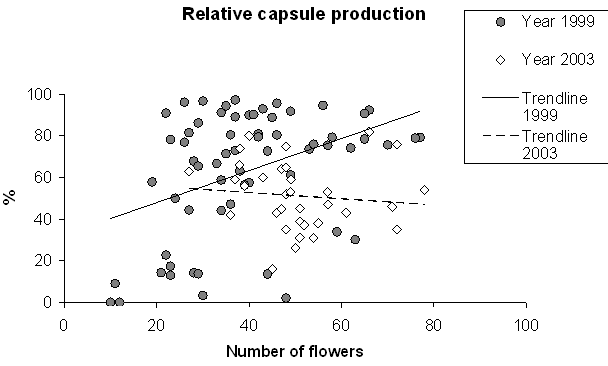 Fig. 5. The relationship between inflorescence size and number of capsules produced in two years in one population of Gymnadenia conopsea.
Fig. 5. The relationship between inflorescence size and number of capsules produced in two years in one population of Gymnadenia conopsea.
|
Discussion
G. conopsea plants with a large inflorescence attracted more flower visitors than plants with a small inflorescence. Large floral displays have been shown to enhance pollinator attraction in several other species (e.g. Schmid-Hempel and Speiser, 1988; Andersson, 1991, Ohara and Higashi, 1994; Thompson, 2001). However, most of the visitors to G. conopsea inflorescences were flies which probably had no effect as pollinators. The proportion of butterfly species capable of pollinating G. conopsea flowers from all visitors was surprisingly low. We found a trend indicating that the large inflorescences are more favoured by actual pollinators. However, in this study, we monitored only day-active butterflies, and thus are unable to assess the behaviour of the nocturnal moths which have been shown to be important pollinators of G. conopsea at least in some populations (Lang, 1989; Pridgeon et al., 2001). In the comparison of the paired plants for pollinator attention, those with larger inflorescences were favoured by visitors. The advantage of producing a large inflorescence was shown in relative capsule production and in dry weight of capsules. Female reproductive success of G. conopsea is most probably regulated both by pollination success and by resource availability in which large plants are superior to small ones. A combination of pollination and resource limitation has been shown to affect within-year reproduction of at least one rewarding orchid species, Platanthera bifolia (Mattila and Kuitunen, 2000).
A large inflorescence with many nectar-producing flowers may lengthen the visitation times of pollinators (e.g. Klinkhamer and de Jong, 1993). Surprisingly, we found no differences in visitation times or in the number of visited flowers between small and large inflorescences of G. conopsea. In contrast to our prediction, the probability of geitonogamous pollination was not higher in large inflorescences. In their experiment separating the effects of flower number from other traits related to plant size, Brody and Mitchell (1997) found in hummingbird pollinated Ipomopsis aggregata that pollinator visitation did not increase disproportionally with flower number. Butterfly visits to G. conopsea were generally very short in duration, which decreases the probability of geitonogamy, since the movement of pollinia from one flower to another requires a relatively long visit by the pollinator. This is because pollinia of Gymnadenia conopsea are initially attached to a pollinator in an upright position and then swivel to point forward in a proper angle for a proper stigma contact (Lang, 1989). The time-gap between the attachment of a pollinium to a pollinating insect and the reaching of a position needed for fertilization may be longer for nectar-producing than for nectarless orchids (Johnson and Nilsson, 1999).
None of the possible negative effects of a large inflorescence on reproductive success found in many other species were detected in G. conopsea. In G. conopsea there was a trend to a greater predation on large inflorescences. However, there was no difference in the relative seed predation of small and large inflorescences. Based on this result, it seems that in the studied population, pre-dispersal seed predation was not enough to limit inflorescence size in G. conopsea. The benefits of receiving more pollinator visits and consequently producing more fruits when producing a large inflorescence are greater than the costs caused by slightly increased seed predation. However, as we had data only from two seasons, we cannot say much about the possible variation in yearly seed predation intensity. Ehrlén (1996, 1997), for example, found in Lathyrus vernus (L.) Bernh. (Fabaceae) that the risk of seed predation varied between years, and there was no consistent relationship between inflorescence size and the role of seed predation in different years. More studies are needed on perennial orchids to examine the relationship between inflorescence size and pre-dispersal seed predation in several consecutive years.
Capsule production, quantity of seed produced and the proportion of embryonic seeds did not differ between geitonogamous and xenogamous flowers. Consequently, the rare event of geitonogamous pollination could lead to normal capsule and seed production. A long history of isolation and a small number of individuals in the study population was shown to decrease within-population genetic variation in the closely related Gymnadenia odoratissima (Gustafsson and Sjögren-Gulve, 2002). Small population size leads inevitably to reproduction between close relatives that may purge deleterious alleles from the population (e.g. Kirkpatrick and Jarne, 2000), even though effectiveness of purging depends on species and population properties (Byers and Waller, 1999). However, the negative effects of inbreeding may become visible later, in seed germination or in growth rate of seedlings.
A large inflorescence seems to be an advantage for G. conopsea plants without any major immediate costs. The species is, however, long-lived and iteroparous, and it would be reasonable to think that there are some factors, that might not be apparent in a one year study, that limit the number of flowers produced by G. conopsea. The growth of terrestrial orchids is fairly slow in most cases, and plant size, which is usually strongly correlated with flower number, is thus limited by age and by site quality.
The reproductive effort of every individual is important for the survival of isolated and small plant populations. Due to low number of inflorescences and often unfavourable spatial distribution of the flowering individuals, these populations often suffer from problems in pollinator attraction. G. conopsea is a non-clonal and out-crossing species, and therefore it needs an efficient device by which to attract pollinators. In this paper, we show that, at least in the short run, it is advantageous for this species to allocate much of available resources to the production of a large floral display. This is because such an allocation will be paid back in the form of a significantly enhanced rate of pollinator visitation. The profitability of producing a high number of flowers is enhanced by the fact that a large inflorescence does not entail any immediate reproductive costs. Compared with plants with a small inflorescence those with a large inflorescence may have an advantage in seed production especially if plants with different-sized inflorescences are located close to each other. Differences in reproductive success between individuals may lead to a continuous loss of alleles, which causes increasing problems for the chance of the population to adapt to future environmental changes.
|
Acknowledgements
The authors thank A. Virtanen and R. Arminen for the help in the field, and M. Mutanen for identification of the seed-predating larvae. We also thank K. Duffy, P. Kindlman, T. Kull and J. Stout for valuable comments on the manuscript.
|
Literature cited
Andersson, S., 1991. Floral display and pollination success in Achillea ptarmica (Asteraceae). Holarct. Ecol. 14: 186-191.
Barrett, S.C.H., Harder, L.D., Cole, W.W., 1994. Effects of flower number and position on self-fertilization in experimental populations of Eichornia paniculata (Pontederiaceae). Funct. Ecol. 8: 526-535.
Biere, A., Honders, S.J., 1996. Impact of flowering phenology of Silene alba and S. dioica on susceptibility to fungal infection and seed predation. Oikos 77: 467-480.
Brody, A.K., Mitchell, R. J., 1997. Effects of experimental manipulation of inflorescence size on pollination and pre-dispersal seed predation in the hummingbird-pollinated plant Ipomopsis aggregata. Oecologia 110: 86-93.
Byers, D.L., Waller, D.M., 1999. Do plant populations purge their genetic load? Effects of population size and mating history on inbreeding depression. Annu. Rev. Ecol. Syst. 30: 479-513.
Calvo, R.N., 1990. Inflorescence size and fruit distribution among individuals in three orchid species. Am. J. Bot. 77: 1378-1381.
Cibula, D.A., Zimmerman, M., 1984. The effect of plant density on departure decisions: testing the marginal value theorem using bumblebees and Delphinium nelsonii. Oikos 43: 154-158.
Ehrlén, J., 1996. Spatiotemporal variation in predispersal seed predation intensity. Oecologia 108: 708-713.
Ehrlén, J., 1997. Risk of grazing and flower number in a perennial plant. Oikos 80: 428-434.
Fægri, K., van der Pijl, L., 1979: The principles of pollination ecology. Pergamon Press, Oxford.
Goulson, D., Stout, J.C., Hawson, S.A., Allen, J.A., 1998. Floral display size in comfrey, Symphytum officinale L. (Boraginaceae): relationships with visitation by three bumblebee species and subsequent seed set. Oecologia 113: 502-508.
Gustafsson, S., Sjögren-Gulve, P., 2002. Genetic diversity in the rare orchid, Gymnadenia odoratissima and a comparison with the more common congener, G. conopsea. Cons. Genet. 3: 225-234.
Hansen, I., Olesen, J.M., 1999. Comparison of reproductive success in two orchids: the nectarless Dactylorhiza majalis s.s and the nectar-producing Gymnadenia conopsea s.l. Nord. J. Bot. 19: 665-671.
Harder, L.D., Barrett, S.C.H., 1995. Mating cost of large floral displays in hermaphrodite plants. Nature 373: 512-515.
Herrera, C.M., 2000. Measuring the effects of pollinators and herbivores: evidence for non-additivity in a perennial herb. Ecology 81: 2170-2176
Hessing, M.B., 1988. Geitonogamous pollination and its consequences in Geranium caespitosum. Am. J. Bot. 75: 1324-1333.
Johnson, S.D., Nilsson, L.A., 1999. Pollen carryover, geitonogamy, and the evolution of deceptive pollination systems in orchids. Ecology 80: 2607-2619.
de Jong, T.J., Waser, N.M., Klinkhamer, P.G.L., 1993. Geitonogamy: The neglected side of selfing. Trends Ecol. Evol. 8: 321-325.
Kirkpatrick, M., Jarne, P., 2000. The effects of a bottleneck on inbreeding depression and the genetic load. Am. Nat. 155: 154-167.
Klinkhamer, P.G.L., de Jong, T.J. 1993: Attractiveness to pollinators: a plant’s dilemma. Oikos 66: 180-184.
Lang, D., 1989. Wild orchids of Great Britain and Ireland. Oxford University Press, Oxford.
Leimu, R., Syrjänen, K., Ehrlén, J., Lehtilä, K., 2002. Pre-dispersal seed predation in Primula veris: among-population variation in damage intensity and selection on flower number. Oecologia 133: 510-516.
Mattila, E., Kuitunen, M.T., 2000. Nutrient versus pollination limitation in Platanthera bifolia and Dactylorhiza incarnata (Orchidaceae). Oikos 89: 360-366.
Molau, U., Eriksen, B., Knudsen, J.T. 1989. Predispersal seed predation in Bartsia alpina. Oecologia 81: 181-185.
Moore, D.M., 1980. Orchidaceae. In: Tutin, T-G., Haywood, V.H., Burges, N.A., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., (Eds), Flora Europaea, Volume 5 Alismataceae to Orchidaceae (Monocotyledones). Cambridge University Press, Cambridge, pp. 325-350.
Myers, J.H., 1985. Effect of physiological condition of the host plant on the ovipositional choice of the cabbage white butterfly, Pieris rapae. J. Anim. Ecol. 54: 193-204.
Nazarov, V., and U. Buchsbaum. 2004. Widderchen als Bestäuber von Orchideen in Thüringen (Insecta: Lepidoptera, Zygaenidae). Entomofauna. 25: 365-372. (in German)
Ohara, M., Higashi, S., 1994. Effects of inflorescence size on visits from pollinators and seed set in Corydalis ambigua (Papaveraceae). Oecologia 98: 25-30.
Pellmyr, O., 2002. Pollination by animals. In: Herrera, C.M., Pellmyr, O. (Eds), Plant-animal interactions. Blackwell Science Ltd, TJ International Ltd, Padstow, Cornwall, UK, pp. 153-184.
Pridgeon, A.M., Cribb, P.J., Chase, M.W., Rasmussen, F.N. (Eds), 2001. Genera Orchidacearum, Volume 2 Orchidoideae (Part one). Oxford University Press Inc, New York.
Raijmann, L.E.L., van Leeuwen, N.C., Kersten, R., Oostermeijer, J.G.B., den Nijs, H.C.M., Menken, S.B.J., 1994. Genetic variation and outcrossing rate in relation to population size in Gentiana pneumonathe L. Cons. Biol. 8: 1014-1026.
Rodríguez-Robles, J.A., Meléndez, E.J., Ackerman, J.D., 1992. Effects of display size, flowering phenology, and nectar availability on effective visitation frequency in Comparettia falcata (Orchidaceae). Am. J. Bot. 79: 1009-1017.
Schmid-Hempel, P., Speiser, B., 1988. Effects of inflorescence size in Epilobium angustifolium. Oikos 53: 98-104.
Schoen, D.J., Dubuc, M. 1990. The evolution of inflorescence size and number: a gamete-packaging strategy in plants. Am. Nat. 135: 841-857.
Shykoff, J.A., Bucheli, E., 1995. Pollinators visitation patterns, floral rewards and the probability of transmission of Microbotryum violaceum, a venereal disease of plants. J. Ecol. 83: 189-198.
Thompson, J.D., 2001. How do visitation patterns vary among pollinators in relation to floral display and floral design in a generalist pollination system? Oecologia 126: 386-394.
Vrieling, K., Saumitou-Laprade, P., Cuguen, J., van Dijk, H., de Jong, T., Klinkhamer, T.J., 1999. Direct and indirect estimates of the selfing rate in small and large individuals of the bumblebee pollinated Cynoglossum officinale L. (Boraginaceae). Ecol. Lett. 2: 331-337.
Wyatt, R., 1981. The reproductive biology of Asclepias tuberosa: II. Factors determining fruit-set. New Phytol. 86: 375-385.
Wyatt, R., Broyles, S.B., 1994. Ecology and evolution of reproduction in milkweeds. Annu. Rev. Ecol. Syst. 25: 423-441.
|
published 20.11.2006
|
Citation rule: Vallius E. et al. 2006. Are there fitness advantages associated with a large inflorescence in Gymnadenia conopsea ssp. conopsea? http://www.r-b-o.eu/rbo_public/Vallius_et_al_2006.html
|
© 2006 by Authors
|
| |
| | |
|