|
Pollination Ecology of Lizard Orchid (Himantoglossum caprinum) in Crimea S. P. Ivanov1, A. V. Fateryga1,2, V. V. Kholodov1 |
 |
||||
|
Экология опыления Ремнелепестника козьего (Himantoglossum caprinum) в Крыму С. П. Иванов1, А. В. Фатерыга1,2, В. В. Холодов1 |
|||
|
1 - Vernadskiy Taurida National University, Simferopol, Ukraine, spi2006@list.ru 2 - Nikitskiy Botanical Garden – National Scientific Center, Yalta, Ukraine, fater_84@list.ru |
|||
|
Abstract The data on composition of the pollinators, the rate of visited and pollinated flowers of the nectarless species, lizard orchid (Himantoglossum caprinum) have been given. The rate of pollinated flowers was low; it varied from 1% to 10% (usually 5–6%) in different years and different points of Crimea. Nine bee species from the families Megachilidae (7 species) and Apidae (2 species) have been recorded as flower-visitors which were able to retrieve pollinaria. Bees of the species Megachile ericetorum (generally males of them) were effective pollinators. The rate of visited and pollinated flowers in comparison with density of the bees and flowers of their forage plants were analyzed. The way of attraction of the pollinators to the flowers has been discussed. |
|||
|
Introduction
Himantoglossum caprinum (M. Bieb.) K. Koch is a rare nectarless terrestrial orchid species described from Crimean peninsula (Flora of the USSR, 1935). As the modern conception the areal of the species includes Crimea and Caucasus (Golubev, 1996). In Crimea H. caprinum is distributed in mountain part of peninsula primary in foothills and south coast slopes. The main habitats of the species are light juniper and oak forests, shrubby grasslands and steppe slopes. As a rule populations of H. caprinum are not numerous and consist usually of some pieces or several tens specimens (Luks, 1978; Kosykh, Golubev, 1983). The species are abundant only on one of the known localities – in the steppe and shrubby slopes of Lisya Bay in the vicinities of the Karadag Nature Reserve (eastern part of Crimean South Coast). Certain years in this area the population of H. caprinum runs to more than thousand generative specimens (Mironova, 2007). Flower ecology of H. caprinum is bad-known. In Crimea it were previously studied only phenology of the blossoming (Mironova, 2007; Ivanov et al., 2008). The species usually blossoms from the last week of May to the middle of the second part of June. Also it is known that the pollination rate of the species usually is low (Ivanov et al., 2003). Any reliable data on the pollinators of H. caprinum was previously unknown except the notation in the Red Book of Ukraine (2009) that the pollinators of this species were bumblebees. In this paper we give the results of our investigations of pollinators, rate of flower visits and pollination of the species in different conditions in Crimea. Materials and methods We carried our investigations in 2007, 2009 and 2010 years in two localities: Ayan Tract (fig. 1a) in the vicinities of Perevalnoye village of Simferopol district (Crimean Foothills) and Lisya Bay (fig. 1b). Some additional data were given in previous years (1992–2005) in Ayan Tract and two points of Sevastopol district (Maksimova Dacha settlement and Chernaya River). One bee specimen with pollinarium of H. caprinum, collected in Krasnolesye village (Simferopol district), was found in entomological collection of Vernadskiy Taurida National University.  Fig. 1. Habitat sites, inflorescences and pollinators of Himantoglossum caprinum: a – habitat site in Ayan Tract, b – habitat site in Lisya Bay, c, d – inflorescences of Himantoglossum caprinum in Lisya Bay, e – male of Megachile ericetorum with pollinaria of Himantoglossum caprinum, frontal view, f – female of Megachile ericetorum with pollinaria of Himantoglossum caprinum, lateral view. In the localities under study we counted the number of blossoming specimens of H. caprinum, measured their height above the ground level, counted the number of flowers per specimen and measured the distance between blossoming plants. Also we measured the density of melittophilic plants flowers (or anthodia in case of composites) by the method of transects (5 parallel transects 1×20 m). At the same time, the density of bees was counted by sweep net (300–900 sweeps). To find more flower visitors of H. caprinum carrying pollinaria of this species we also carried out individual capture of bees on their feed plants flowers. At the end of blossoming terms we checked all flowers in the sample plants and recorded four conditions of them: non-visited flowers (pollinarium is present, stigma is without massulae), flowers visited first time (pollinarium is absent, stigma is without massulae), pollinated flowers (stigma with massulae) with pollinarium and pollinated flowers without one. Then we counted the rate of pollinated flowers and the rate of flowers visited first time and estimated the index of flower visits’ repetition by means of dividing rate of pollinated flowers by rate of flowers visited first time (Ivanov, Kholodov, 2003). This index is equal to average number of flowers visited by pollinator after a visit of the first one. Also we carried out measurements of the main flowers parameters such as length of the spur and width of the corolla throat and main parameters of the flower-visiting bees such as length of the proboscis and width of head near the center of the clypeus. All measurements were done by the ocular scale bar of the binocular MBS-9. Plants names are cited according to nomenclature checklist of vascular plants of Ukraine (Mosyakin, Fedoronchuk, 1999). Names of bee taxa correspond to the classification of C. D. Michener (2007). Description of blossoming conditions In Lisya Bay the coenopopulation of H. caprinum grew in association Elytrigietum (nodosae) festucosa (rupicolae) – teucriosum (chamaedrytis) with projective cover from 60% to 90%. Number of blossoming specimens in the studied site varied from 25 to more than 100 in different years. Height of the plants varied from 27 to 78 cm (48.6±4.8 in average, n=31, p=0.05). Number of flowers per plant was from 8 to 59 (27.5±2.9 in average, n=52, p=0.05) (fig. 1c, d). Seventeen species of melittophilic plants were blossoming in the studied site simultaneously with H. caprinum. Among them the most abundant were the following: Teucrium chamaedrys L. (9.8–82.7 flowers/m2), Inula germanica L. (1.9–17.4 flowers/m2) and Bupleurum rotundifolium L. (1.7–25.2 flowers/m2). Orchid specimens grew diffusely with 37.4±6.6 cm (n=85, p=0.05) average distance of one another. In the studied site it were registered 32 bees species belonged to families of Megachilidae (18 species), Apidae (12 species) and Halictidae (2 species). In Ayan Tract the coenopopulation of H. caprinum grew in association Inulieto (asperae) – Filipenduletum (vulgaris) caricetum with projective cover from 95% to 100%. Number of blossoming specimens in the studied site varied from 12 to 65. Height of the plants was nearly just like in Lisya Bay – from 29 to 80 cm (47.6±4.2 in average, n=29, p=0.05). But in the same time inflorescences were significant sparser: number of flowers per plant was from 5 to 30 (16.3±1.7 in average, n=59, p=0.05). In this site 23 species of melittophilic plants were blossoming simultaneously with H. caprinum. The most abundant species were following: Galium rubioides L. (155.6–480.0 flowers/m2), Dorycnium herbaceum Vill. (32.9–1,960.0 flowers/m2) and Melilotus officinalis (L.) Pall. (17.0–334.0 flowers/m2). Plants of H. caprinum grew here sparser than in Lisya Bay: average distance between specimens was 91.6±21.2 cm (n=61, p=0.05). Twenty eight species of bees were recorded in the studied site, among them 6 species belonged to Megachilidae, 7 – to Apidae, 8 – to Halictidae, 4 – to Andrenidae, 2 – to Colletidae and 1 – to Melittidae. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Pollinators and pollinaria attaching
All recorded specimens of flower-visitors of H. caprinum belonged to long-tongued bees (table 1): 7 species of the family Megachilidae (first 14 specimens) and 2 species of the family Apidae. Only Megachile ericetorum bees (6 specimens) carried 3 and 4 pollinaria per specimen (2 in one case), two specimens of two species carried 2 pollinaria each and other 8 specimens of 6 species carried 1 pollinaria each or only viscidia remained from one. Flower visiting was observed only in one case with female of Megachile parietina (not included in the table). The bee flew up to an inflorescence with some hovering and slightly rapider landed to one of the flowers. Then she had inserted her proboscis into the spur and flew out. The pollinarium did not been retrieved because it had been already retrieved by a previous visitor of this flower.
Generally pollinaria of H. caprinum was attached to the bees on the center part of the clypeus or near the frontal-clypeal suture, seldom on the front (fig. 1e, f; fig. 2c, d). Pollinaria attached to the apical margin of the clypeus recorded in Anthidium loti and in both specimens of Megachile pilicrus. To retrieve the pollinarium a bee must touch the bursicula with frontal surface of the head. The bursicula cover the viscidium located closely above the stigma (fig. 2a, b). If pollinarium were attached to the clypeal margin, massulae will not touch the stigma after reconfiguration of pollinarium. Instead of this they will touch a throat surface near the spur orifice. Thus A. loti and M. pilicrus are not able to pollinate flowers of H. caprinum. All other species are theoretically able to pollinate the orchid but pollinaria with spent massulae recorded only in M. ericetorum. Males of this species have perfect morphological compliance with H. caprinum flowers. They have proboscises with 4.3–7.4 mm long (6.10±0.30 mm in average, n=29, p=0.05) and heads with 2.3–3.9 mm wide (3.02±0.12 mm in average, n=29, p=0.05). Orchid flowers have spurs with 6.5–11.4 mm long (9.09±0.36 in average, n=35, p=0.05) and corolla throats with 2.4–3.7 mm wide (2.99±0.11 in average, n=35, p=0.05). Thus width ot the head varies in the same diapason with the corolla throat and the proboscis is significantly shortly than the spur. In Lisya Bay, 2007 we recorded some flowers of H. caprinum with operated spurs. Probably they was not been operated by recorded flower visitors but by bumblebee Bombus argillaceus (Scopoli, 1763) or by three species of Xylocopa, which were very abundant in this territory. 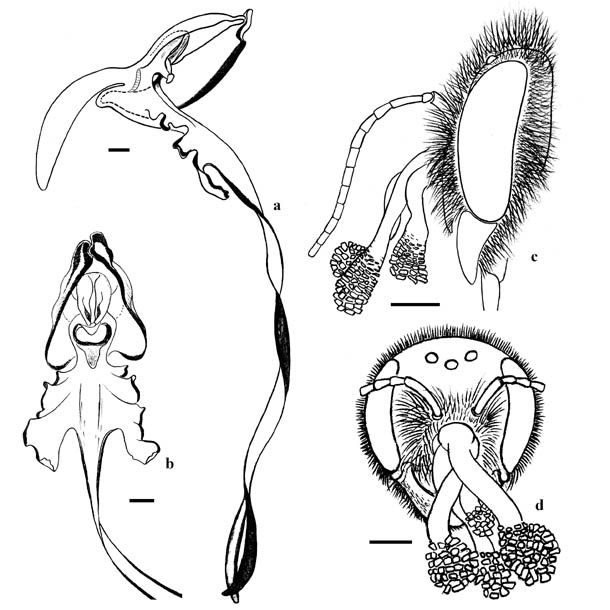 Fig. 2. Flowers of Himantoglossum caprinum and location of pollinaria on bee heads: a – lateral view of the dissected flower, b – frontal view of the flower, c – lateral view of Anthidium cingulatum male head with pollinaria, d – frontal view of Megachile ericetorum female head with pollinaria. All scale bars – 1 mm.
Fig. 2. Flowers of Himantoglossum caprinum and location of pollinaria on bee heads: a – lateral view of the dissected flower, b – frontal view of the flower, c – lateral view of Anthidium cingulatum male head with pollinaria, d – frontal view of Megachile ericetorum female head with pollinaria. All scale bars – 1 mm.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Pollination effectiveness The data on pollinated and visited flowers rate in different bees’ density and bees’ forage flowers density were summarized in table 2. The rate of pollinated flowers was low and varied from slightly more than 2% to more than 10%. In other years and in other studied localities we obtained the similar data. In Ayan Tract the rate of pollinated flowers was 1.4 (in 2005) and 3.6% (in 2008). In Maksimova Dacha it was 0.6% (in 1993), 3.4% (in 1995), 5.5% (in 1992) and 6.8% (in 2000). In Chernaya River the rate of pollinated flowers in only studied year (1993) was 6.5%.
The rate of pollinated flowers in two localities individually correlates with the density of bees, especially long-tongued (table 2). In Lisya Bay the highest rate of pollinated flowers was in 2007 when the highest density of long-tongued bees had been registered. Also that year it was recorded the highest rate of flowers visited first time. The high rate of such flowers indicates an abundance of non-specialized flower visitors which can retrieve pollinaria but can not pollinate flowers, or visit flowers only once (low repetition of flower visits). Also the high rate of flowers visited first time corresponds with the very low flower density of bees forage plants. In two other years the density of flowers was higher and the density of bees was lower, thus the rates of visited and pollinated flowers were lower too. In Ayan Tract the density of flowers was far higher than in Lisya Bay but the density of long-tongued bees was comparable. Thus the rates of visited and pollinated flowers were comparable too. The high repetition of flower visits (especially in 2010) suggests that among long-tongued bees inhabited in this locality the specialized pollinator (M. ericetorum) predominated. Discussion Among all species of orchids the mellitophilic pollination syndrome is the most widespread (Cherevchenko et al., 2010). The bees are attracted to the flowers by nectar or pseudopollen rewards; by generalize imitation of food sources; by imitation of food sources confirmed with floral mimicry; by giving a places for sleeping; by imitation of bee females (sexual-deception) and by imitation of bee males (pseudoantagonism). The generalized imitation of food sources is the most widespread among melittophilic orchids’ species. Flowers of these species have not any visible similarity with flowers of rewarding sympatric species. Generalized food-deceptive orchids get effective pollination when they blossom in segregation from other plant species with nectar reward. It is achieved by the phenological separation between orchids and rewarding plants: the former usually flower earlier than the latter (Ivanov et al., 2008; Pellissier et al., 2010). H. caprinum has no similarity with flowers of rewarding species (Ivanov et al., 2003) but in Ayan Tract it begin flower a month later after the first generalized food-deceptive species (e.g. Orchis simia Lam.) and blossom amidst the higher density of rewarding species. Also it has the lowest rate of pollinated flowers while other six species of orchids with this attractive mechanism have one about 35–60% (Ivanov et al., 2008). Thus H. caprinum adapt to existence with low pollination rate which is provided by some peculiar mechanisms of attraction the pollinators. Megachilids are seldom recorded as the effective pollinators of nectarless orchids. In Europe the bees of this family (oligolectic species of the genus Chelostoma) were recorded only as the effective pollinators of red helleborine (Cephalanthera rubra (L.) Rich.) which had mimicry with the bees forage flowers of the genus Campanula (Nilsson, 1983; Nazarov, Ivanov, 1990). H. caprinum is the second species pollinating by megachilids, and like in C. rubra it is pollinating by one or few closed species. Predominance of the males in flower visitors may suggest an idea that flowers of the species are sexual-deceptive. But presence of a few females, several pollinaria with spent massulae in female of M. ericetorum and observed behavior of M. parietina are the evidences of a food-deception. This deception is generalized, without floral mimicry and flower visitors are mostly polylectic. Concrete factors which attract on the flowers mostly megachilids (especially M. ericetorum) and not other bees are unknown. Probably it can be a special olfactory attractant which is efficacious only to megachilids. This hypothesis is not such unreal because food-deceptive species of the closely related genus Steveniella apparently emit attractant which is efficacious only to diplopterous wasps, especially of the subfamily Vespinae (Nazarov, 1995). Specific olfactory attractants for vespids are also recorded in rewarding species, broad-leaved helleborine (Epipactis helleborine (L.) Crantz) (Brodmann et al., 2008). Among non-orchid rewarding plants water betony (Scrophularia umbrosa Dumort.) attracts on its flowers different vespids but some species (mostly males of them) are attracted strongly and become “sluggish” after many repeated visits (Fateryga et al., 2006). The males and the female of M. ericetorum also demonstrate persistently repeated visits of H. caprinum flowers: all of them carried several pollinaria and the most of them had spent massulae. One case with the male without spent massulae on his three pollinaria suggests that he had visited three flowers in such a short time before the reconfiguration of the pollinaria happened. So H. caprinum adapt to bee pollination and most probably emit a special olfactory attractant for megachilids, especially for some morphologically suitable species, e.g. M. ericetorum. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Literature
Brodmann J., Twele R., Francke W., Ayasse M. Pollinator-attracting semiochemicals of the wasp-flower Epipactis helleborine // Mitt. Deutsch. Ges. Allg. Ang. Entomol. 2008. Bd. 16. S. 171-174. Cherevchenko T. M., Buyun L. I., Kovalska L. A. Pollination strategies in orchids (Orchidaceae) // Ukrayinsky Botanichny Zhurnal [Ukr. Bot. J.]. 2010. Vol. 67. № 5. P. 637-649. (In Ukrainian). Fateryga A. V., Ivanov S. P., Novikov Y. V. Vespid wasps (Hymenoptera: Vespidae) as specialized pollinators of a rare figwort species, Scrophularia umbrosa (Scrophulariales: Scrophulariaceae) in the Crimea // Isvestiya Kharkovskogo Entomologicheskogo Obshchestva [Kharkov Entomol. Soc. Gazet.]. 2006. Vol. 14. № 1-2. P. 145-161. (In Russian). Flora of the USSR / Ed. V. L. Komarov. Leningrad, 1935. Vol. 4. 760 p. (In Russian). Golubev V. N. Biological flora of Crimea / 2nd ed. Yalta, 1996. 126 p. (In Russian). Ivanov S. P., Fateryga A. V., Tyagniryadno V. V. Comparative evaluation of orchids pollination effectiveness in natural boundary Ayan // Bulleten Nikitskogo Botanicheskogo Sada [Bull. Nikitskiy Bot. Garden]. 2008. Iss. 97. P. 10-14. (In Russian). Ivanov S. P., Kholodov V. V. Analysis of nectarless orchids (Orchidaceae) pollination pattern subject to their spatial placing // Points on the development of the Crimea. Simferopol, 2003. Iss. 15. P. 57-65. (In Russian). Ivanov S. P., Kobechinskaya V. G., Oturina I. P., Pilipenko N. V. Dynamics flowering and pollination efficiency of unnectarous and nectarous kinds of orchids in Crimea // Pytannya Bioidykatsiyi ta Ecologiyi [Questions of Bioindication and Ecology]. 2003. Iss. 8. № 2. P. 43-50. (In Ukrainian). Kosykh V. M., Golubev V. N. Modern state of populations of the rare, vanishing and endemic plants of Mountain Crimea. Yalta, 1983. 119 p. (In Russian). Lukss Y. A. To orchid classification of the Crimean flora by rarity categories // Bulleten Nikitskogo Botanicheskogo Sada [Bull. Nikitskiy Bot. Garden]. 1978. Iss. 3. P. 15-18. (In Russian). Michener C. D. The bees of the World / 2nd ed. Baltimore, 2007. xvi + 953 p. Mironova L. P. Rare species // Chronicle of nature (of the Karadag Nature Reserve). Simferopol, 2007. P. 87-140. (In Russian). Mosyakin S. L., Fedoronchuk M. M. Vascular plants of Ukraine: a nomenclature checklist. Kiev, 1999. xxiii + 345 p. Nazarov V. V. Pollination of Steveniella satyrioides (Orchidaceae) by wasps (Hymenoptera, Vespoidea) in the Crimea // Lindleyana. 1995. Vol. 10. № 2. P. 109-114. Nazarov V. V., Ivanov S. P. Pollination of mimetic species Cephalanthera rubra (Z.) Rich. and Campanula taurica Juz. by bees of the genus Chelostoma Latr. (Hymenoptera, Megachilidae) in the Crimea // Entomologicheskoye Obozrenie [Entomol. Rew.]. 1990. Vol. 69. № 3. P. 534-537. (In Russian). Nilsson L. A. Mimesis of bellflower (Campanula) by the red helleborine orchid Cephalanthera rubra // Nature. 1983. Vol. 305. № 5937. P. 799-800. Pellissier L, Vittoz P., Internicola A. I., Gigord L. D. B. Generalized food-deceptive orchid species flower earlier and occur at lower altitudes than rewarding ones // J. Plant Ecol. 2010. Vol. 3. № 4. P. 243-250. Red book of Ukraine. Plant kingdom / Ed. Y. P. Didukh. Kyiv, 2009. 912 p. (In Ukrainian). |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
republished 5.10.2011 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Published in: I. I. Shamrov (ed.). Okhrana i kultivirovaniye orkhidey [Protection and cultivation of orchids] (Materials of the 9th International conference, St.-Petersburg, September 26–30, 2011). – Moscow: KMK Scientific Press Ltd., 2011. – P. 187–194.
Опубликовано в: Охрана и культивирование орхидей (Материалы IX Международной конференции, Санкт-Петербург, 26–30 сентября 2011 г.). – Москва: Товарищество научных изданий КМК, 2011. – С. 187–194. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
© 2011 by Authors |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||